Discuss the complications of toxins and dermal fillers and provide an evidence based protocol for immediate, short term and long-term management of these complications.

Dr MJ Rowland-Warmann BSc BDS MSc Aes.Med. PGDip Endod. MJDF RCS (Eng)
For her MSc Masters Degree in Aesthetic Medicine for the Queen Mary University of London. The following essay received a Distinction – the highest mark awarded.
This is an academic essay. If you are a patient seeking Botox® treatment from one of Liverpool’s most learned aestheticians then take a look at our main treatments page here: Botox Liverpool & Lip Fillers Liverpool. For information on getting lips dissolved with hyalase follow the link.

Toxins and Fillers are associated with a number of serious complications. Over 40 cases of blindness have already been reported in the literature following dermal filler injections. Anti-aging medicine has seen a sharp rise in popularity in recent years. In 2013, 1.5 million toxin and filler treatments were carried out in the UK, a rise of 4% more than in 2012 (33). Both are noninvasive and have high rates of patient satisfaction (1, 6).
Most complications can be avoided by a thorough knowledge of patient, procedure and relevant anatomy in addition to assessing patient expectations. Even when complications arise, it is imperative that practitioners are equipped to treat, intervene and manage adverse events (7). This commentary will focus on complications arising from Botulinum Toxin (BT) and non-permanent dermal fillers such as Hyaluronic Acid dermal (HA), Calcium Hydroxylapatite (CaHA) and Poly-L-Lactic Acid (PLLA), the most frequently utilised products in modern aesthetic medicine.
Botulinum Toxins (Botox®)
Botulinum Toxin (BT) is used in the treatment of hyperdynamic facial rhytides. In its 20 year history, BT has demonstrated product safety with minimal downtime post procedure (15, 28). The duration of action of BT is around 4 months with no permanent effects (1,8)
Dermal Fillers
More than 200 types of dermal filler products are currently on the market (6). Fillers are biodegradable and have a duration of action of 6-18 months on average with a favourable side effect profile (6, 27). All fillers have different indications for use and the injector must know their own and the product’s limitations. Hyaluronic Acid (HA) fillers are the most popular owing to their ease of use and reversibility (23). Calcium Hydoxylapatite (CaHA) and Poly-L-Lactic Acid (PLLA) fillers are irreversible and their effects can last for 18 months to 2 years, respectively (11, 27)
Complications
Complications can be defined as an unfavourable evolution of treatment. Minimising complications includes conducting a thorough examination and checking a patient’s medical history, thorough record keeping and post-procedure instruction to the patient. Complications may be self-limiting or require intervention to limit and manage them (2). Whilst it may not be feasible to consider every possible complication, a thorough process will create a more predictable outcome. An example of how to minimise complications is shown in Figure 1.
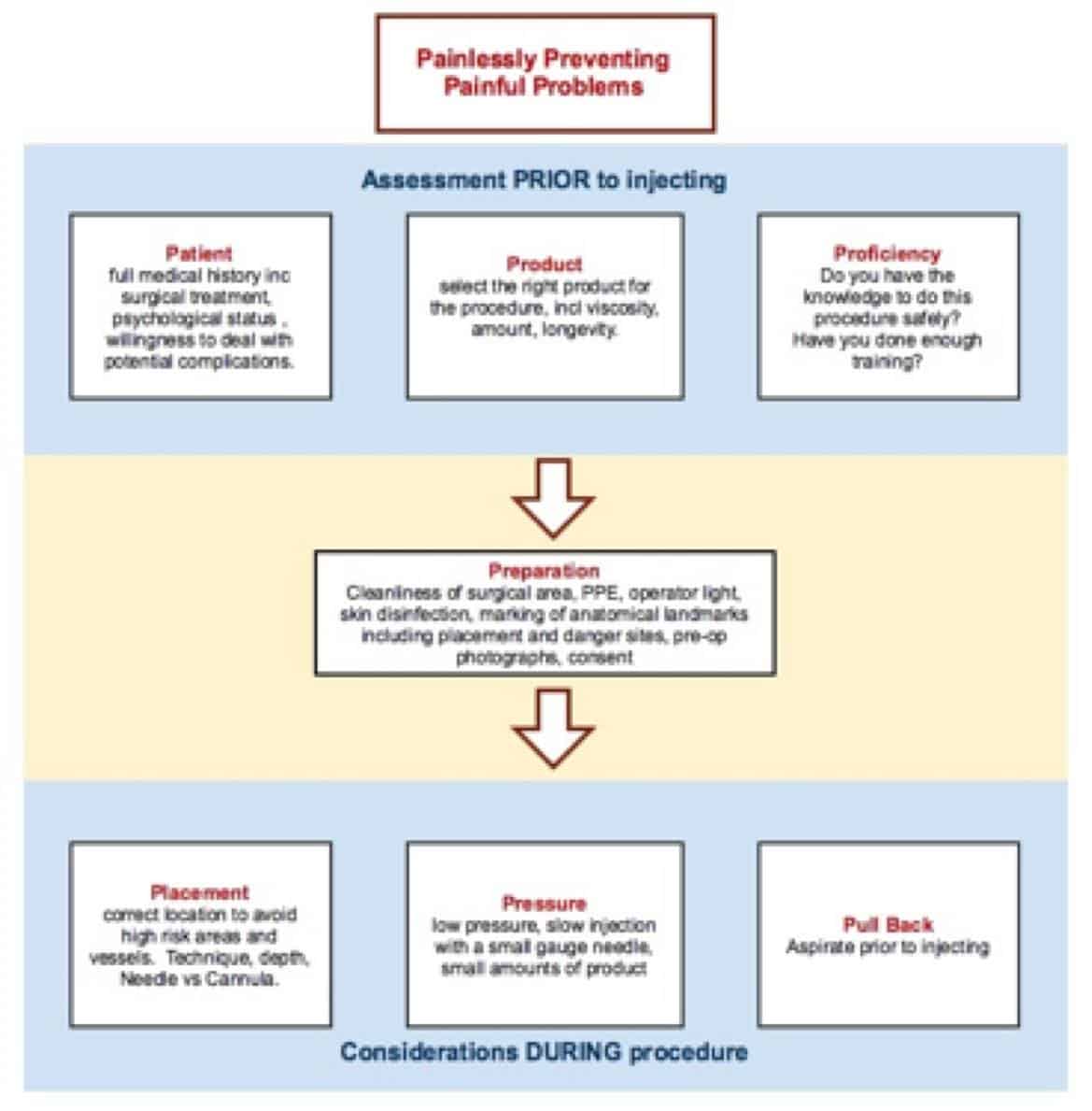
Figure 1: Protocol for complication prevention at Smileworks (Liverpool).
Combined Complications of Botox and Filler
Bruising, Swelling and Erythema
These are the most common early complications seen with both BT and filler treatment and often expected. Where vessel perforation occurs during treatment causing a haematoma, firm pressure should be applied for five minutes (5). The patient should be reassured that bruising, swelling and erythema are short lived likely sequelae. Post procedure cooling will reduce bruising and swelling, which usually resolves within one week.
All patients should be reviewed after two weeks in order to follow up and be encouraged to inform the practitioner of any concerns. Several medications increase the risk of bleeding (3). Whilst some reports advise discontinuing blood thinning medications, the practitioner should always be aware of the reason for the prescription and consider the risk to the patient should these drugs be stopped (30,33). It must always be remembered that these are purely elective procedures, and the best way to avoid any complications is not to treat.
Allergy and Anaphylaxis
A serious complication of BT or filler treatment is an allergic reaction or anaphylaxis. Modern dermal fillers do not routinely require allergy testing and Hypersensitivity to HA is 0.6-0.8% (30). Additionally diluents used in BT treatment may elicit allergic responses (34). Delayed hypersensitivity reactions are mostly self-limiting, occasionally requiring oral steroid or antihistamines (4), but anaphylaxis is a medical emergency and must be dealt with immediately per resuscitation council guidelines (35).
Complications of Botulinum Toxin (Botox)
Due to its short lived action, complications of BT treatment are generally transient. Nevertheless, these can be distressing for patient and practitioner alike.
Headaches
A common post-treatment occurrence, headaches are thought to be procedure rather than product-related (15). Patients should be reassured and, if necessary, advised to take suitable analgesia (28).
Unwanted effects on non-target muscles
Eyelid or brow ptosis results from inappropriate deactivation of muscle groups responsible for facial expression. Spread of BT to adjacent muscle groups paralyses them (3). Brow ptosis occurs when the frontalis muscle is over-treated, resulting in decreased ability to raise the brow. This should be treated with reassurance and review, little will remedy the situation except waiting for the product to wear off. Certainly no more product should be added.
An eyelid ptosis with loss of voluntary eye closure is caused by diffusion of product into the levator palpebrae superioris muscle, causing involuntary closure of the lid. Both are distressing to the patient, who complains of heavy eyelids and fatigue (17). Lid ptosis often becomes apparent several days post treatment, the first line treatment is with 5mg/ml iopidine (apraclonidine) eye drops to act on Muller’s muscle, contracting it and elevating the lash margin (8,17). A successful treatment, this gives an immediate but transient effect and should be maintained daily until the effects of the BT have diminished.
Diplopia can occur as a result of BT diffusion into the lateral rectus muscle. Due to the nature of the dose of diffusion and the limited product duration in areas of high muscle use, patients should be reassured and reviewed until the effects have decreased. In some cases patients should refrain from driving until ocular effects diminish. An exaggeration of the arch of the eyebrow can occur if the lateral parts of the frontalis are insufficiently treated and the medial sections are dosed to paralysis. To remedy this quizzical expression, addition of units to the lateral frontalis is advised (34).
A good rule to prevent the above is to inject 1-2cm from the orbital rim for corrugator and orbicularis oculi, and 2.5cm above the brow for treatment of frontalis (3, 29). The ‘frozen face’, although fashionable is not a desired outcome and the dosage should be adjusted to minimise this recurring. This complication is temporary and the patient should be reassured and reviewed (28).
Muscle paralysis outside the scope of treatment is common. This is due to diffusion of the product into sites not intended for treatment; the duration of action of the undesired effect is limited due to the low dose of the offending diffusion (33). Consensus advise caution in treating the mid face, lower face and neck due to unpredictable outcomes (29).
Undercorrection
Some patients return claiming BT treatment has been unsuccessful. This can be managed by counselling the patient on a realistic outcome pre-procedure with consideration of the patient’s skin ageing (15). If the treatment has not had the desired cosmetic effect due to under-treatment, then the practitioner may elect to re-treat (34)
Complications of Filler Treatment
Complications of filler may be transient or require intervention. Whilst bruising and swelling is self-limiting, the following complications are likely to require management. The longer duration the injected product, the higher the rate of complication is likely to be (14).
Inappropriate Placement
Placement of the product too superficially can result in filler being visible under the skin or the appearance of nodules (3,27). When HA filler placement is superficial, the Tyndall effect can result, reflecting light giving a blue hue to the tissue (27). The remedy for this is massage in the first instance, followed by dissolution of the product with Hyaluronidase if unsuccessful (21, figure 5). Visible nodules of CaHA and PLLA are more difficult to remove if placed superficially, as this filler does not respond to hyaluronidase. In this case massage and review is preferable prior to referral for excision in addition to following any guidelines given by the manufacturer (28,30).
Infection and biofilms
Preparation of the skin prior to treatment, preferably with 2% Chlorhexidine, is essential (30). However, skin pathogens such as Staphylococcus aureus are tenacious and can be inoculated into the skin via the temporary disruption of the epidermal barrier during treatment (7, 22). Infection of filler sites can be immediate, occurring within days of treatment, or long term, resulting in painful inflammatory nodules. If the patient presents with redness, swelling and pain within two weeks, treatment should be for infection of the treatment site with antibiotics (31). The suggested treatment is 500mg Clarithromycin with 400mg Moxifloxacin initially, changing to Clindamycin 600mg plus Tetracycline 500mg if no improvement within 3 days. Treatment with antibiotics may be required for up to 4 weeks (30, 33).
Biofilms are late-onset infections around filler deposits (3), encapsulating bacteria in an extracellular matrix (7). They can become chronic and are more common in longer lasting fillers; Hyaluronidase can be used to disrupt the biofilm in order to allow penetration with antibiotics if sole antibiotic treatment is unsuccessful although this runs the risk of spread of infection and potential cellulitis (4,25). If a biofilm is suspected, referral to a specialist centre is advisable as bacterial cultures will be necessary to eliminate it.
Granulomas
A granuloma can occur in response to any filler and it is understood that all dermal fillers cause soft-tissue reactions and fibrosis (28). Prior to treating any suspected granuloma, the occurrence of which is 0.02-1% in filler treatment, the nodule should be treated as a suspected infection to prevent possible infection spread in the first instance (32). The risk of granuloma increases with the longevity of the filler and occurs as a response to frustrated healing (20, 22, 27) and is higher in stimulatory products such as CaHA and PLLA (7), causing palpable or visible nodules (3).
With HA fillers, granulomas may be dispersed with Hyaluronidase (22). Methods employed for non-HA fillers include massage or disruption of the capsule. An excellent protocol for this is utilising a mixture of the antimitotic agent 5-Fluouracil (5FU) 0.5ml of 50mg/ml, Triamcinolone 0.3ml of 10mg/ml and 0.2ml 2% Lidocaine, and injecting 0.1ml to 0.5ml into the nodule (20,27). The fibrous capsule around the filler is firm and resistance to injection will be felt. Once the resistance subsides, it is advisable to stop further injection as cortisone can cause tissue atrophy. If this method is unsuccessful, referral for excision is advised (20). It is sometimes difficult to distinguish between treatment of infection and granuloma. Figure 2 highlights steps to remedy lumps and bumps occurring after filler treatment.
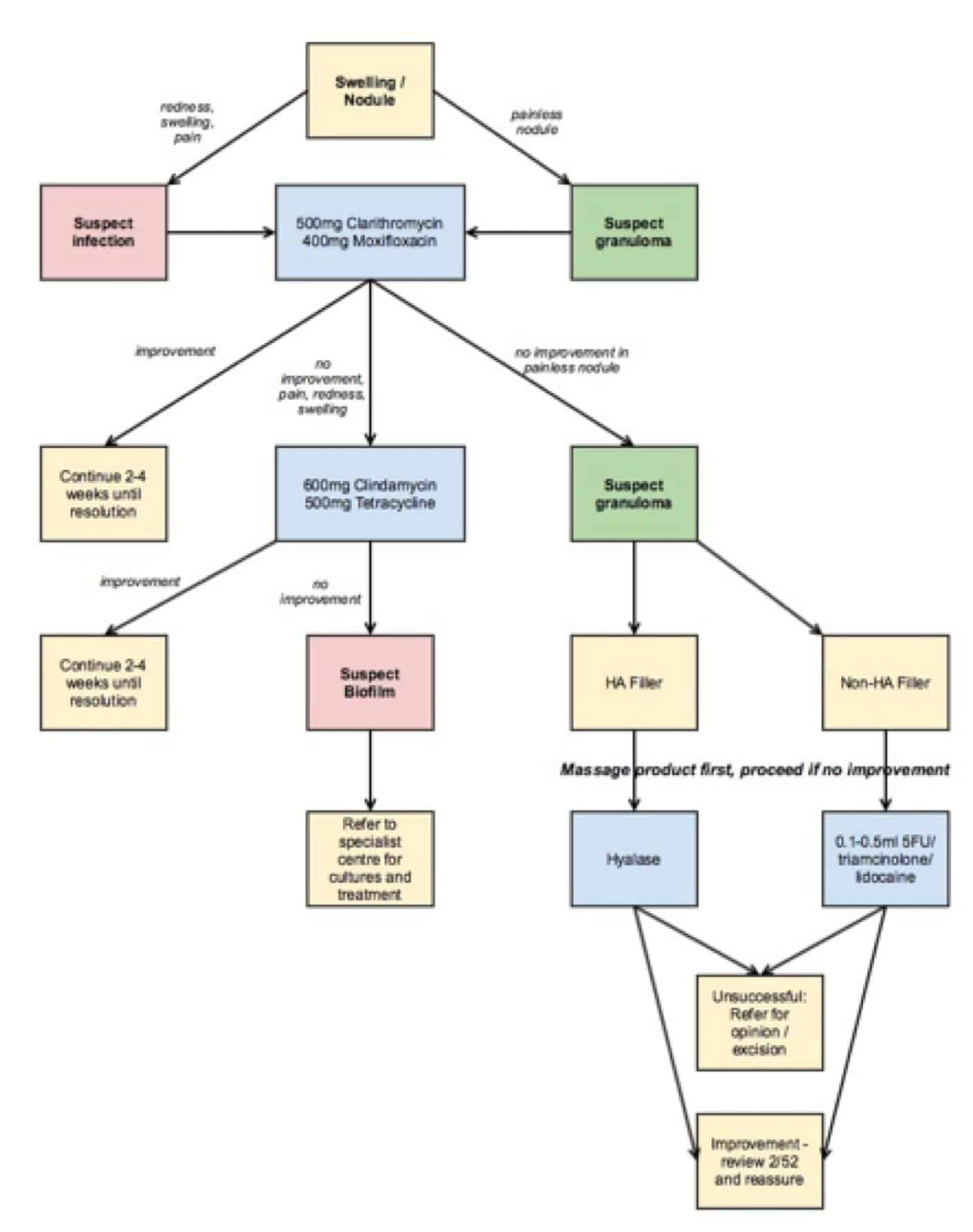
Figure 2: Treatment algorithm for infections and biofilms
Vascular complications
Vascular complications are rare but serious. They occur by direct occlusion of a vessel via product injection, embolisation of product in a distant vessel, or compression of vessels by injecting product adjacent to them (6). The resulting effects can lead to tissue ischaemia, necrosis or blindness depending on the affected structures (10,14). Figure 3 illustrates high risk areas of the face.
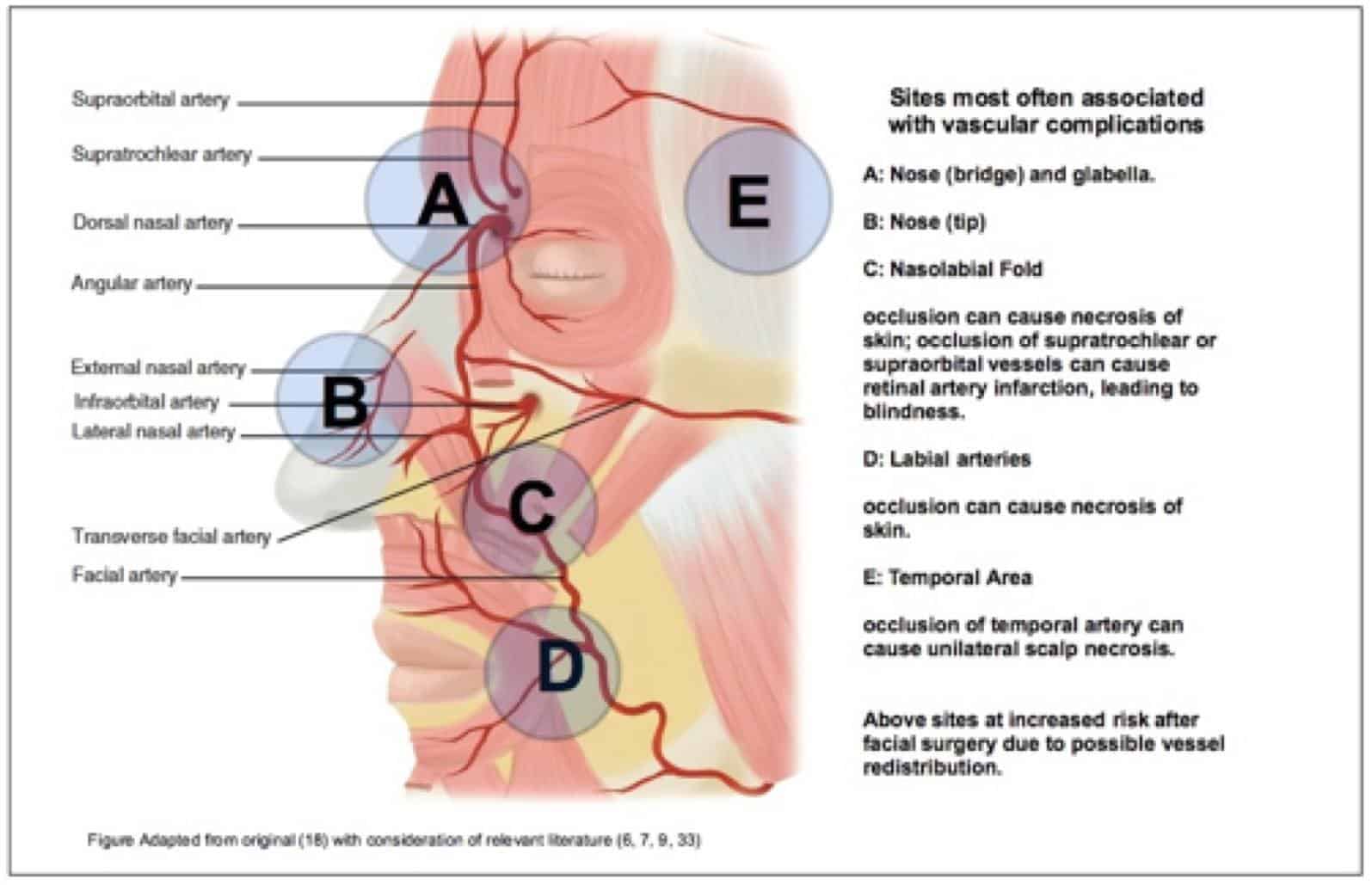
Figure 3: High risk areas of the face
The immediate signs of impending necrosis are skin blanching or reticulated violaceous patches along the vessel distribution lasting more than 2-3 minutes often, and often but not necessarily with increased procedural pain (4,33).
The procedure must be stopped and a warm compress applied to the affected area to increase vasodilation. GTN patches or 2% nitroglycerin paste should be applied to the skin whilst preparing Hyalase, which should then be injected into and around the affected area. Whether HA or other filler products have been used, Hyalase should be employed – degradation of endogenous HA may decompress vessels and bring about some resolution even in non-HA filler. After injection of Hyalase, the product should be massaged to encourage dissolution for 5-10 minutes. Aspirin 325mg is useful at this stage to prevent further clotting (6,9,33).
In the event of full recovery, 75mg Aspirin daily is recommended for 2 weeks with regular review (3). Prednisolone 20-40mg for 3-5 days is of benefit (6). If there is little improvement, oxygen should be given and referral to A&E considered. If there is no improvement or worsening of the patient’s condition, an urgent referral to A&E should be made (4,6). Any ocular involvement is a serious matter which requires urgent referral to a specialist centre (24, 26).
If the patient has suffered necrosis already – in instances where the initial compromise was not recognised or the onset was late, wound care is needed to minimise the consequences. This includes silicone dressing and twice weekly reviews until the skin has healed (9). Figure 4 illustrates management of vascular complications.
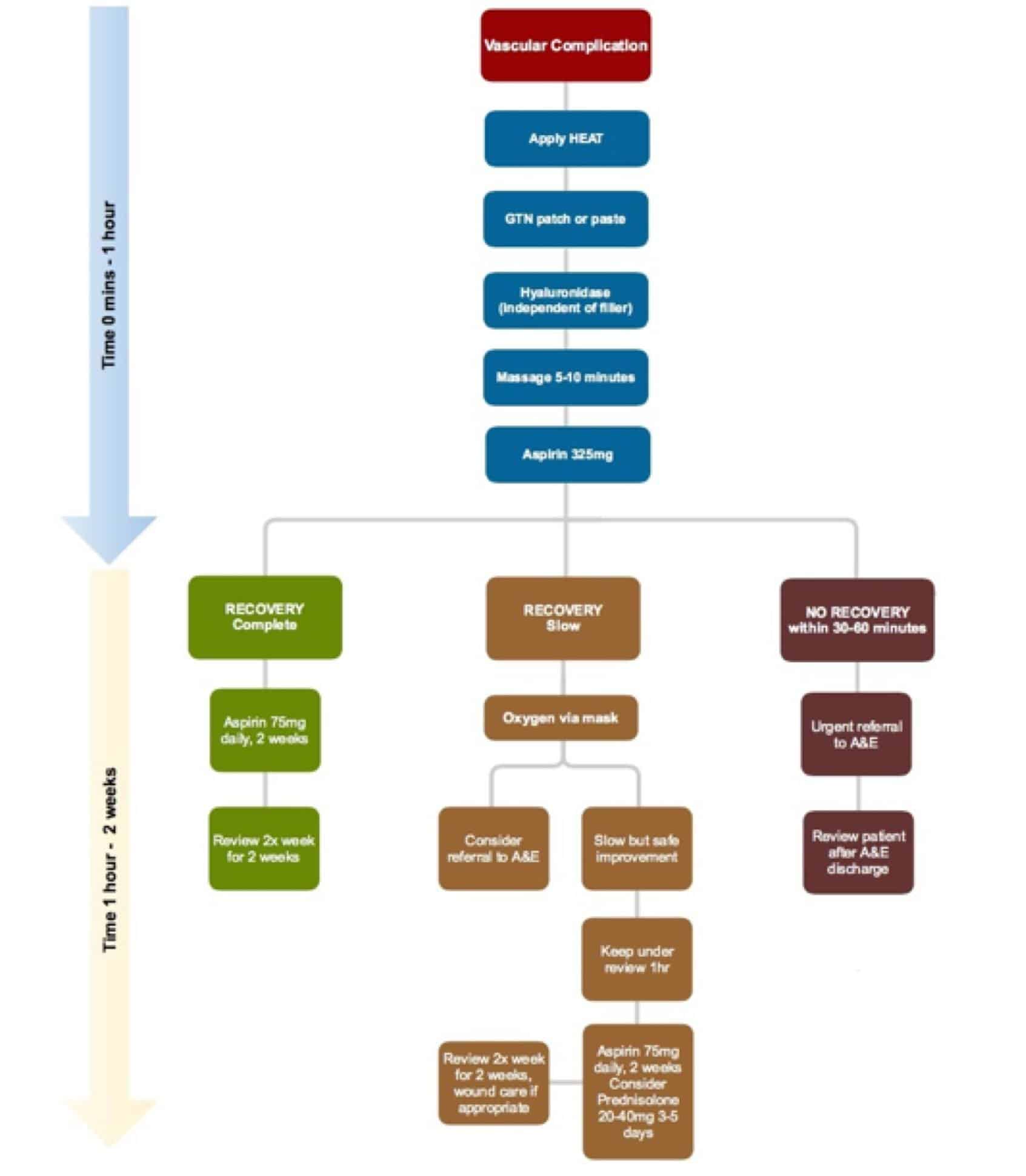
Figure 4. The management of vascular complications. (References: 3,6,9,33)
Hyaluronidase
Hyaluronidase is a naturally occurring endoglycosidase which can effect the degradation of HA (12, 32). Its use in aesthetic dermatology is off-label (33). Derived from ovine source, Hyalase is available in the UK as a 1500iu/vial powder.
An algorithm for the use of Hyalase in both corrective and urgent aesthetic settings is set out in figure 5 (12, 16, 32).

Figure 5 (References: 12, 16, 33)
Hyalase has carries an allergy risk of approximately 0.05-0.69% and has numerous drug interactions (32,33). Patients should be counselled that a depression may form at the location of the filler as Hyalase degrades endogenous hyaluronic acid, a constituent of GAG naturally found in the skin (12).
Conclusion
Complications are sometimes inevitable events and patients should be well informed of the possibility of adverse events and it is advisable not to treat those who are not understanding of possible sequelae (2). Dealing with complications means that practitioners need to be fully equipped for the job. It is not simply syringes of toxin and filler that are essential, but also hyaluronidase and a medical emergencies kit akin to that set out by the resuscitation council. This means the practitioner can adequately take control of the situation at hand.
The true prevalence of adverse events is unknown (25). Oftentimes practitioner embarrassment hinders a reliable database and many complications stay unreported. The ease of use and reversibility of HA fillers is also their danger; in the UK their use is almost unlicensed and those injecting are often doing so after day-long courses with a poor understanding of the technique. In addition, this can take place in their patient’s kitchens and lounges, ill-equipped to deal with poor outcomes (1).
Eliminating complications by effective management starts at prevention and ends with a satisfied, safe patient. The journey in between must contain all steps that can be reasonably made to ensure patient safety.
References
- Klein AW; Contraindications and Complications with the use of botulinum toxin. Clinics in Dermatology (2004)22, 66-75
- Sherman RN; Avoiding Dermal Filler Complications. Clinics in Dermatology (2009)27, S23-S32
- Emer J, Waldorf H; Injectable neurotoxins and fillers: There is no free lunch. Clinics in Dermatology (2011)29, 678-690
- Ozturk CN, Li Y, Tung R, Parker L, Peck Piliang M, Zins JE; Complications following injection of soft-tissue fillers. Aesthetic Surgery Journal (2013)33, 862-877
- Alam M, Dover JS; Management of Complications and Sequelae with Temporary Injectable Fillers. Plastic and Reconstructive Surgery (2007)120, 98S-105S
- Beleznay K, Humphrey S, Carruthers JDA, Carruthers A; Vascular Compromise from Soft Tissue Augmentation. Journal of Clinical and Aeshtetic Dermatology (2014)7, 37-43
- Daines SM, Williams EF; Complications Associated with Injectable Soft-Tissue Fillers. JAMA Facial Plastic Surgery (2013)15, 226-231
- Klein AW; Complications, Adverse Reactions, and Insights with the use of Botulinum Toxin. Dermatological Surgery (2003)29, 549-556
- Glaich AS, Cohen JL, Goldberg LH; Injection Necrosis of the Glabella: Protocol for Prevention and Treatment after use of Dermal Fillers. Dermatologic Surgery (2006)32, 276-281
- Park SW, Woo SJ, Park KH, Huh JW, Jung C, Kwon O; Iatrogenic Retinal Artery Occlusion Caused by Cosmetic Facial Filler Injections. American Journal of Ophthalmology (2012)154, 653-662
- Broder KW, Cohen SR; An Overview of Permanent and Semipermanent Fillers. Plastic and Reconstructive Surgery (2006)118, 7S-14S
- Lee A, Grummer SE, Kriegel D, Marmur E; Hyaluronidase. Dermatological Surgery (2010)36, 1071-1077
- Hyalase Product information https://products.sanofi.com.au/aus_pi_hyalase.pdf. Accessed January 2015.
- Tracy L, Ridgway J, Nelson JS, Lowe N, Wong B; Calcium hydroxylapatite associated soft tissue necrosis: A case report and treatment guideline. Journal of Plastic, Reconstructive and Aesthetic Surgery (2014)67, 564-568
- Ascher B, Talarico S, Cassuto D, Escobar S, Hexsel D, Jaen P, Monheit GD, Rzany B, Viel M; International consensus recommendations on the use of botulinum toxin type A (Speywood Unit) – part 1: upper facial wrinkles. Journal of the European Academy of Dermatology and Venerology (2010)24, 1278-1284
- Hirsch R, Cohen JL, Carruthers JDA; Successful Management of an unusual presentation of impending necrosis following a hyaluronic acid injection embolus and a proposed algorithm for management with hyaluronidase. Dermatological Surgery (2007)33, 357-360
- Karami M, Taheri A, Mansoori P; Treatment of botulinum toxin-induced eyelid ptosis with anticholinesterases. Dermatological Surgery (2007)33, 1392-1395
- Grunebaum LD, Bogdan Allemann I, Dayan S, Mandy S, Baumann L; The risk of alar necrosis associated with dermal filler injection. Dermatological Surgery (2009)35, 1635-1640
- Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M, Zimmermann U, Duffy DM; Foreign body granulomas after all injectable dermal fillers: part 1. Possible Causes. Plastic and Reconstructive Surgery (2009)123, 1842-1863
- Lemperle G, Gauthier-Hazan N; Foreign body granulomas after all injectable dermal fillers: Part 2. Treatment Ooptions. Plastic and Reconstructive Surgery (2009)123, 1864-1873
- Sclafani AP, Fagien S; Treatment of injectable soft tissue filler complications. Dermatological Surgery (2009)35, 1672-1680
- Alijotas-Reig J, Fernandez-Figueras MT, Puig L; Inflammatory, immune-mediated adverse reactions related to soft tissue dermal fillers. Seminars in Arthritis and Rheumatism (2013)43, 241-258
- Kim DW, Yon ES, Ji YH, Park SH, Lee BI, Dhong ES; Vascular complications of hyaluronic acid fillers and the role of hyaluronidase in management. Journal of Plastic, Reconstructive and Aesthetic Surgery (2011)64, 1590-1595
- Hsieh YH, Lin CW, Huang JS, Yeh PT; Severe ocular complications following facial calcium hydroxylapatite injections: two case reports. Taiwan Journal of Ophthalmology (2014)0, 1-4
- Beer K, Avelar R; Relationship between delayed reactions to dermal fillers and biofilm: facts and considerations. Dermatological Surgery (2014)0, 1-5
- Lazzeri D, Agostini T, Figus M, Nardi M Pantaloni M, Lazzeri S; Blindness following cosmetic injections to the face. Plastic and Reconstructive Surgery (2012)129, 995-1012
- Funt D, Pavicic T; Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clinical, Cosmetic and Investigational Dermatology (2013)6, 295-316
- Cox SE, Adigun CG; Complications of injectable fillers and neurotoxins. Dermatologic Therapy (2011)24, 524-536
- Carruthers J, Fagien S, Matarasso SL, Botox Consensus Group; Consensus Recommendations on the use of Botulinum Toxin Type A in Facial Aesthetics. Plastic and Reconstructive Surgery (2004)114, 1-24
- Bailey SH, Cohen JL, Kenkel JM; Etiology, Prevention and Treatment of Dermal Filler Complications. Aesthetic Surgery Journal (2011)31, 110-121
- DeLorenzi C; Complications of injectable fillers, Part 1. Aesthetic Surgery Journal (2012)33, 561-575
- Cavallini M, Gazzola R, Metalla M, Vaienti L; The role of hyaluronidas in the treatment of complications from hyaluronic acid dermal fillers. Aesthetic Surgery Jounal (2013)33, 1167-1174
- Inglefield C, Collins F, Duckett M, Goldie K, Huss G, Paun S, Williams S; Expert consensus on complications of botulinum toxin and dermal filler treatment. Aesthetic Medicine Expert Group (2014) Second Edition.
- Niamtu J; Complications in Fillers and Botox. Oral and Maxillofacial Surgery (2009)21, 13-21
- https://www.resus.org.uk/pages/anaalgo.pdf, accessed 26.01.15